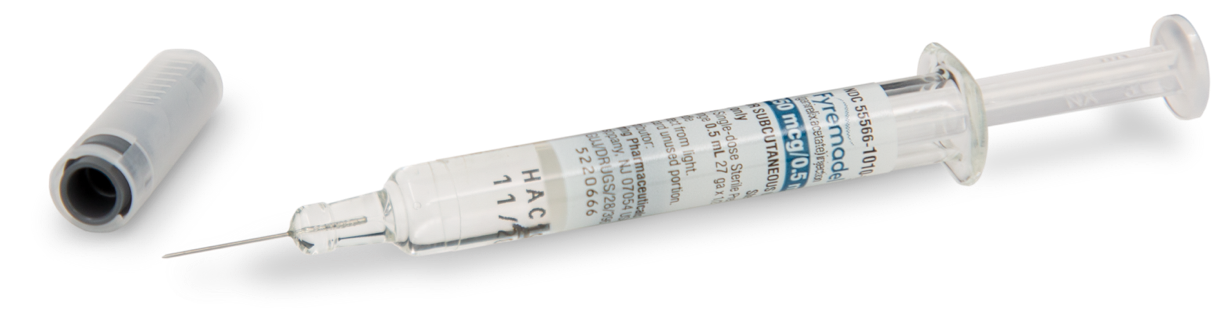
FYREMADEL® (ganirelix acetate) injection is used to prevent surges of luteinizing hormone (LH) in women undergoing controlled ovarian hyperstimulation. Premature surges in LH can cause ovulation to occur too early.1

FYREMADEL has high antagonistic activity against naturally occurring gonadotropin-releasing hormone (GnRH) receptors.1

FYREMADEL is rapidly absorbed following subcutaneous injection.1
FYREMADEL comes in a convenient, ready-to-use, prefilled glass syringe, and does not require refrigeration.1
Reference
1. FYREMADEL® (ganirelix acetate) injection. Prescribing Information. Ferring Pharmaceuticals Inc.
FYREMADEL® (ganirelix acetate) injection
INDICATION FOR USE
- FYREMADEL® (ganirelix acetate) injection is indicated for the inhibition of premature LH surges in women undergoing controlled ovarian hyperstimulation.
IMPORTANT SAFETY INFORMATION
- Ganirelix acetate injection is contraindicated under the following conditions: Known hypersensitivity to ganirelix acetate or to any of its components, Known hypersensitivity to GnRH or any other GnRH analog, Known or suspected pregnancy.
- Ganirelix acetate injection should be prescribed by physicians who are experienced in infertility treatment. Before starting treatment with ganirelix acetate, pregnancy must be excluded. Safe use of ganirelix acetate during pregnancy has not been established.
Please see
Important Safety Information
and
Full Prescribing Information